For the past 40 years, Team Consulting has been a leading partner for drug delivery device development. Trusted by 9 out of the top 10 pharmacos, we help our clients bridge the gap between user and technology, delivering effective device design for different therapeutic areas.
From novel approaches for targeted intratumoural drug delivery, to injection and respiratory devices manufactured at scale, we help our clients develop medical products that stand out in the market and improve patient outcomes.
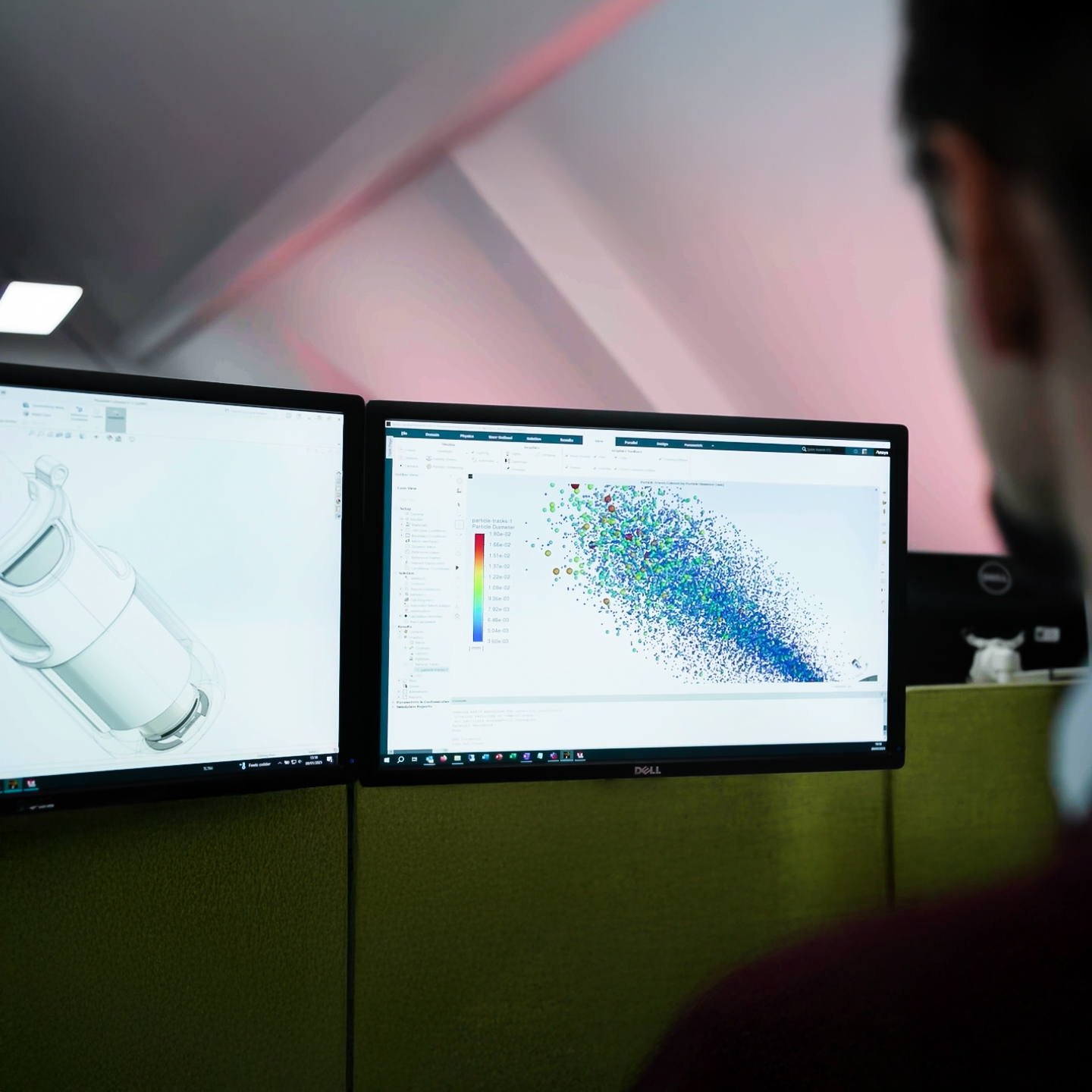
Comprehensive drug delivery device expertise
From opportunity discovery to full-scale industrialisation, we deliver end-to-end drug delivery device development as well as targeted support for standalone work packages. Our experts integrate seamlessly with your team, accelerating your development and helping you solve the hard problems.
Whether you require an independent, expert perspective for device due diligence, strategic input on technology selection, or regulatory support to meet your submission, we bring proven experience and evidence-backed decisions to your project.

An unparalleled mix of multidisciplinary skills
For every project, we draw on expertise from our specialists across systems engineering, design, human factors, manufacturing, device testing, quality engineering and project management – ensuring a seamless, multidisciplinary approach to your development. All our work is governed by a rigorously maintained ISO 13485:2016-certified quality management system, giving you confidence in compliance, reliability and excellence at every stage.

Design for high volume production
High-volume production shouldn’t mean sacrificing effective usability and design. We combine cutting-edge engineering with industrialisation expertise to create drug delivery devices that are both high-performing and manufacturable at scale.
We understand that design, material selection, manufacturing and assembly workflows can all impact the success of your product. Our approach integrates performance reliability and production readiness at every stage of your development, delivering you confidence and market-leading solutions that set new benchmarks in the industry.

Supporting your regulatory approval
Navigating the regulatory landscape for drug delivery devices is complex. Our team has deep expertise in both European and FDA requirements, including several members on ISO committees that actively help to shape the regulations that govern the industry. Our in-depth knowledge of the drug delivery regulations allows us to guide your approval strategy from concept to launch, supporting your approval strategy and helping you to accelerate your path to market.

Sustainable drug delivery solutions
Team has a dedicated sustainability group that challenges the way medical devices are designed, to help you develop a drug delivery device that will mitigate greenhouse gas emissions and limit waste. Applying rigorous Life Cycle Analysis (LCA), we advise on material selection, manufacturing, transportation and more, allowing you to apply sustainable approaches at all stages of your product’s life.

Meet one of our drug delivery experts
Chris has 30 years of experience in the development of medical devices, with a focus on drug delivery: inhalers, injectors and on body delivery systems.
His expertise spans all phases of development from strategy, planning and requirements capture to industrialisation, design transfer and post-market support. Chris regularly presents at international conferences and is a member of ISO committee TC84 for drug delivery devices.
Explore our drug delivery client success stories
Support across the drug delivery landscape

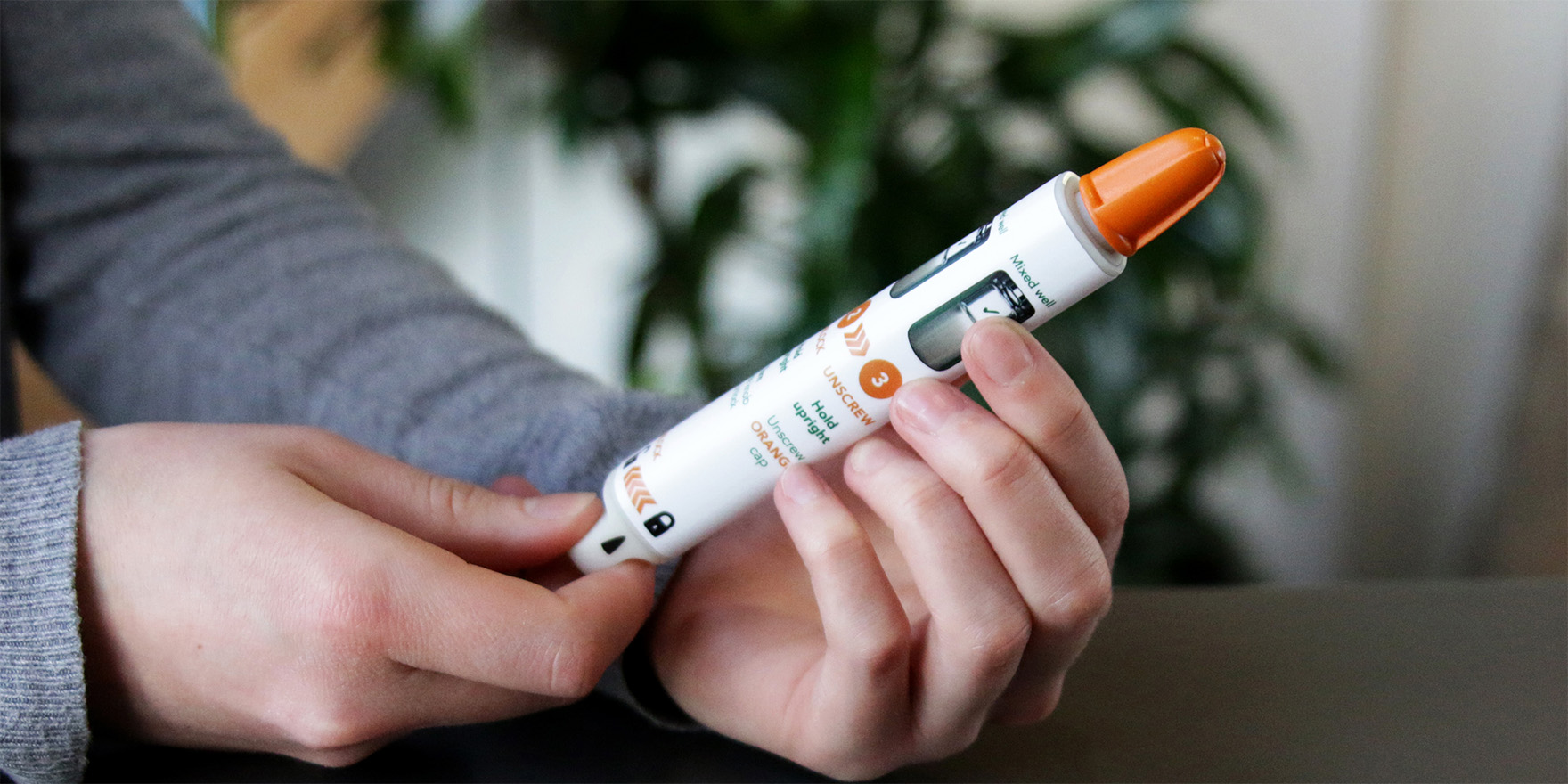
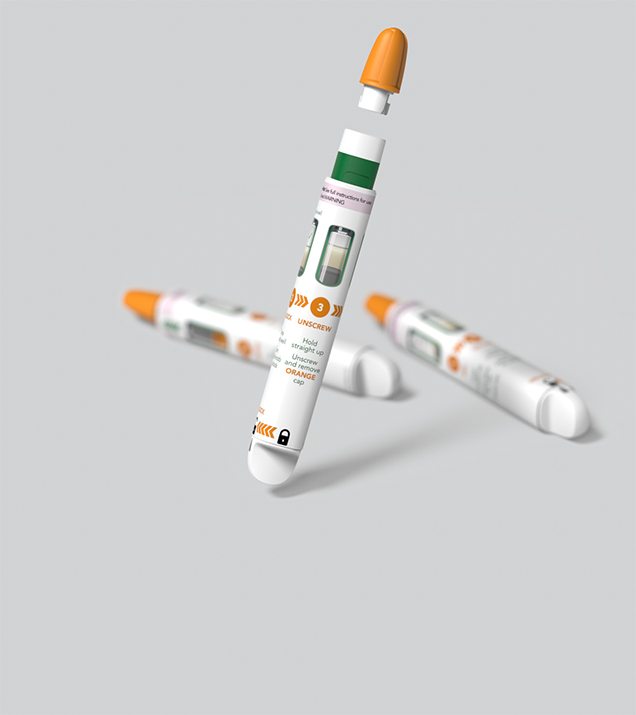
From novel approaches to user-centred, reusable auto-injectors, to emergency-use devices built to five nines reliability, we have an extensive track record in delivering high quality parenterals.
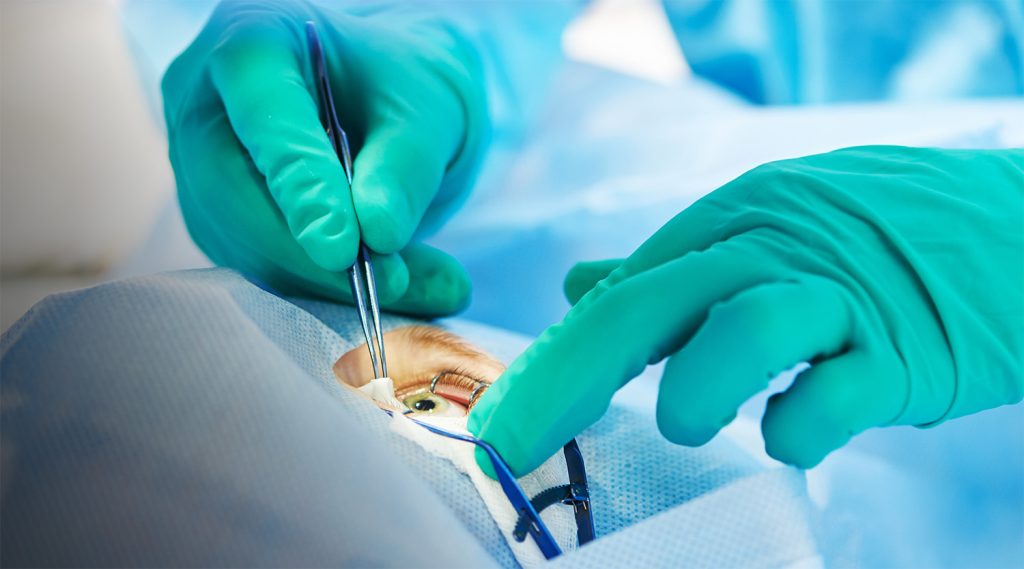
Our experts have supported clients working at the cutting edge of ocular drug delivery, from surgical delivery devices to other novel technologies.
Our applied science experts and mechanical engineers are adept at finding and optimising approaches to targeted drug delivery, from novel injection technologies to cryoablation and more.
Accordion Section
From novel approaches to user-centred, reusable auto-injectors, to emergency-use devices built to five nines reliability, we have an extensive track record in delivering high quality parenterals.
Our experts have supported clients working at the cutting edge of ocular drug delivery, from surgical delivery devices to other novel technologies.
Our applied science experts and mechanical engineers are adept at finding and optimising approaches to targeted drug delivery, from novel injection technologies to cryoablation and more.