Whether you are considering licensing a device to deliver your therapy or looking for an independent assessment of your own product, we have 30 years of experience and technical knowledge to review the performance and robustness of your medical device.
Technical assessment of a medical device
Whatever your challenge, we can build a work programme to help you assess your specific technology and product area. We employ a suite of tools and processes to comprehensively evaluate a device at any stage of its development, such as:
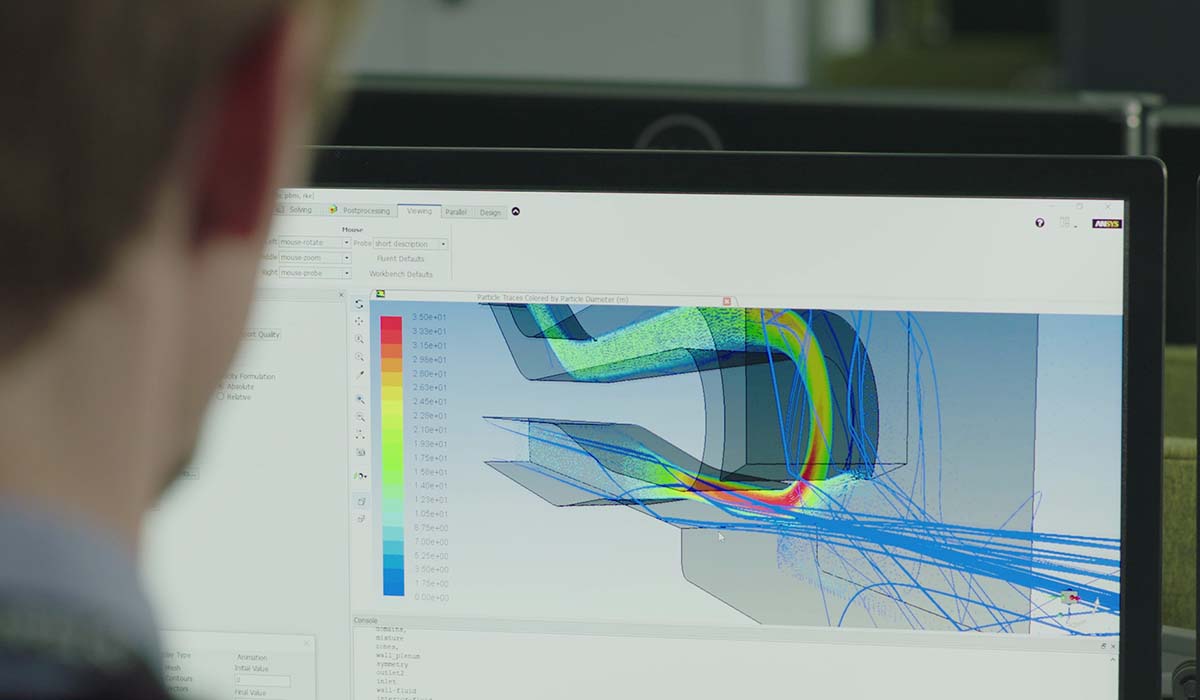
Engineering analysis
Analysis and simulation tools such as math modelling, Finite Element Analysis (FEA) and Computational Fluid Dynamics (CFD) allow us to provide you with a focussed, quantitative assessment of fundamental performance aspects of a device.
Manufacturing review
Formal design for manufacture, assembly reviews, statistical tolerance analysis and cost modelling can provide confirmation – or otherwise – that the system under review is (or could be) viable for your commercial production.
Risk analysis tools
Whether top-down system level risk, fault tree analysis, or bottom-up failure mode and effects analysis, these tools provide robust methodologies for objective assessment of user, design and process risks.
Human factors reviews
We perform desk-based expert reviews from analytical assessments (including product conformity assessment) to small scale user studies. These analyses can provide you with a rich and independent insight into the product’s safety and effectiveness of use.
Established contacts in the industry
As a leader in the medical device field, we have built constructive relationships with key companies across the industry, working closely with their technical and commercial teams. This enables us to have detailed conversations and gain access to the information you need to make a thorough technical evaluation of their products before you commit to licensing.
Meeting your priorities
Our team develop a structured approach when conducting your investigation, to make best use of the time available and ensure we focus on your priorities. Our consultants undertake upfront research and initial evaluation, developing a detailed agenda for discussions, whether it’s a multi-day on-site visit, or a series of online meetings.
Through technical audit activities our experts carefully review the status and robustness of any medical device. Coupled with our experience in device selection and due diligence, we ensure that your target technology meets your needs.

Our capabilities
Team has experienced consultants in all the disciplines needed to audit a product:
- Mechanical engineering
- Electronics and hardware design
- Software
- Verification testing
- Regulatory
- Risk Management
- Human Factors
- Industrialisation








